|
How Does Evaporative Cooling Work?
Evaporative cooling occurs when water evaporates from
a surface. That surface becomes cooler because of the
heat expended by changing a liquid into a vapor.
A nice breeze on a hot day cools us because the current
of air makes persperation evaporate quickly. The heat
needed for this evaporation is taken from our body surfaces.
As water comes in contact with air, it evaporates to
become moisture in the air. The amount of water the
air can hold depends largely on how much water is already
in the air. The term humidity describes the amount of
water in the air. At any given temperature, there is
a maximum amount of water that the air can hold.
What is Relative Humidity?
Humidity is said to be high if the air contains large
amounts of moisture and low if the air contains only
a small amount of moisture. When the air holds as much
moisture as possible at a given temperature, the air
is saturated. The warmer the air, the more moisture
it can hold. Relative humidity (RH), gauges the amount
of water in the air relative to the amount needed for
saturation. If the air contains half the amount of moisture
it can hold, the relative humidity is 50%.
A British Thermal Unit (BTU) is the amount of heat needed
to raise 1 pound of water to 1° F.
In order for water to evaporate, heat is required. To
evaporate one gallon of water requires almost 8,700
BTU's (2,192 kilocalories or .580 kilocalories per gram
of skin temperature) of heat. This heat is taken from
whatever the water is in contact with, cooling the object
as it evaporates. The heat can be taken from your body,
from the air itself, or from garment made from Hydroweave®.
Does Cool Water Absorb More Heat Than Hot Water?
Water temperature does not have a great effect upon
the cooling produced through evaporation. At 90°
F (32° C), it takes 9,000 BTU's to evaporate a gallon
of 50° F (10° C) water, and 8,700 BTU's to evaporate
a gallon of 90° F water. In this example, the water
is 180% warmer and results in only a 3% reduction in
the amount of heat absorbed.
The Higher The Temperature The
Better Hydroweave® Works
The drier the day, the drier the air, the greater the
evaporative cooling effect. As the day gets hotter,
the relative humidity becomes lower and the evaporative
cooling effeciency increases.
Stated another way, RH decreases as air temperature
increases. For every 20° F (11°c) rise in temperature,
the moisture-holding ability of air doubles. For instance,
if the RH is 50% at 70° F (21° C), the RH would
only be 25% at 90° F (32° C).
The extent that relative humidity decreases throughout
the day can be affected by weather systems and proximity
to large bodies of water. If a warm weather system moves
in, but has a lot of water associated with it already,
the decrease in humidity will not be as great.
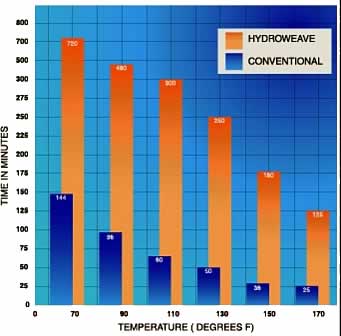
Auburn University tested Hydroweave® to evaluate
how long it cooled. Samples were heated in an oven,
starting at 70° F with 50% RH and gradually increasing
to 170° F amd 1.5% RH. When compared to conventional
fabric, the Hydroweave® samples lasted 5 times longer.
Evaporation
Evaporation is the conversion of a liquid substance
into a gaseous state. This change in state from a liquid
to a gas causes a decrease in the temperature of the
remaining liquid. To maintain the liquid at a constant
temperature, surrounding heat must be absorbed.
Heat Transfer By Vaporization
The body's normal response to heat is sweating. The
secretion and evaporation of sweat is the principal
mechanism by which the human body gets rid of excess
heat.
In the course of doing work, the body generates heat
through its heart, lungs, and muscles. When the air
temperature is higher than body temperature, then radiation,
conduction and convection, all transfer heat into the
body rather than out. The body must overcome this heat
or become overheated, with serious health consequences.
The heat expelled through evaportation can come from
sweat or from surrounding heat sources. The more heat
that is absorbed, the faster evaporation and cooling
occur.
The moisture lost through evaporation creates a second
problem, dehydration. As little as 2% dehydration by
weight can seriously impair a person's ability to react
and think. Physiology texts state that about 600 grams
of insensible moisture loss occurs per day from the
skin.
The effectiveness of evaporative cooling is determined
by the area of the wet surface and by the closeness
of its contact with the dry air. The greater the surface
area, the more effective the cooling. To get the largest
air contact possible, BodyTeq garments made with Hydroweave®
suspend thousands of tiny water absorbing (hydrophilic)
fibers in a field of water resistant (hydrophobic) threads.
Each hydrophilic fiber is held loosely within the batting,
increasing air circulation and the effective wetted
surface.
Definitions
-
Effective Temperature is the combined effect
of temperature, humidity, and air motion on
the body.
-
Dry Bulb Temperature (DB) is the temperature
measured by a thermometer.
-
Wet Bulb Temperature (WB) is the temperature
that can be achieved through the evaporation
of water. It is measured by a psychrometer.
-
Calorie is the unit of heat energy required
to raise the temperature of 1 gram of liquid
water 1° C (from 14.4° C to 15.5°
C)
-
BTU (British Thermal Unit) is the amount
of heat energy required to raise the temperature
of 1 pound of water 1° Fahrenheit (from
59.5° F to 60.5° F)
|
Calculating Cooling
The temperature drop can be calculated. Assuming 80%
effeciency, Hydroweave® will reduce the wet bulb
depression temperature by 80% (the difference between
Wet Bulb and Dry Bulb temperatures).
95 Dry Bulb Temperature
-75 Wet Bulb Temperature
20° Wet Bulb Depression
20° x 80% = Temperature Drop
95° - 16° = 79° Dry Bulb Temperature
Active Evaporative Cooling vs.
Passive Heat-Sink
To demonstrate cooling effectiveness, BodyTeq vests
made with Hydroweave® fabric were tested as a passive
heat sink beneath an encapsulated polyethylene-coated
Tyvek barrier suit. The tests were conducted by Auburn
University and measured the physiological changes of
the wearer (e.g., core temperature) and tolerance times.
In these tests, BodyTeq vests using Hydroweave®
were proven as an effective deterrent to heat stress
while increasing work time by an average of 16.4%.
As these tests demonstrated, Hydroweave® can be
used to make an effective "passive" heat sink.
Now let's examine how much heat can be absorbed. If
it takes 1 BTU to raise one pound of water 1° F
(or 1 calorie to 1 gram one° C) then a cooling vest
with 2 pounds/grams) of water at 70° F (21°
C) in "passive" heat sink mode would absorb
57.2 BTU's (14.512 kilocalories) before reaching 98.6°
F (37° C).
However, heat absorbption characteristics are dramatically
different when evaporation can take place. Again, looking
at heat absorbption, it requires approximately 8700
BTU's to evaporate 1 gallon of water or 2,192 kilocalories
(580 calories/grams). A BodyTeq vest will hold about
1/4 of a gallon or 2 pounds (907 grams) of water and
in "active" evaporative mode will remove 2088
BTU's (526 kilocalories) of heat before all the water
has evaporated.
<<
back
|
